Biden admin “twisted” chemical abortion pill data, HHS Secy.
By: Lisa Bourne, originally published September 4, 2025, Pregnancy Help News
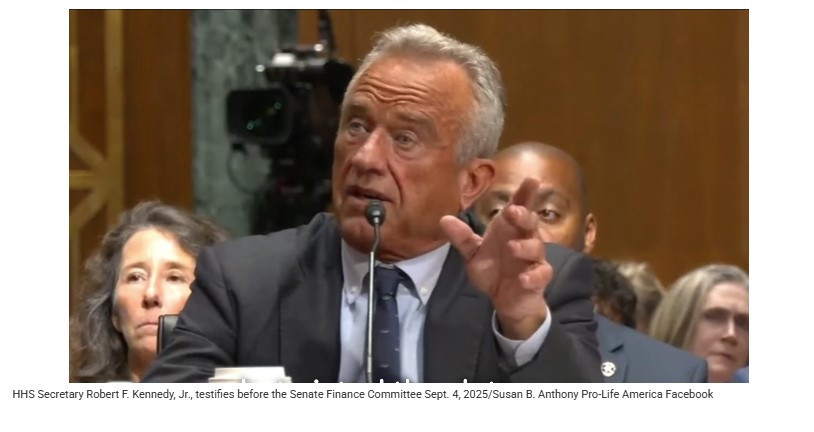
The Biden administration misrepresented data on chemical abortion pill mifepristone to obfuscate safety issues with the drug, Health and Human Services Secretary Robert F. Kennedy, Jr. said Thursday.
Kennedy appeared before the Senate Finance Committee where Oklahoma Sen. James Lankford asked him about ongoing FDA review of safety protocols for mifepristone. Kennedy confirmed that a review was progressing and said that in the process it was determined that the Biden administration had “twisted” the data on mifepristone’s safety.
“We’re getting data in all the time, new data that we’re reviewing, and we know that during the Biden administration, they actually twisted the data to bury one of the safety signals with a very high safety signal of around of 11%,” Kennedy said.
“So, we’re going to make sure that that doesn’t happen anymore,” he added. “We’re producing honest science and gold standard science on that.”
FDA review of mifepristone is hotly contested, with pro-life advocates hoping the federal agency will reinstate the drug’s safety standards and otherwise act to protect women, while abortion proponents claim mifepristone is as safe as Tylenol and argue that safety regulations for the drug impede access.
Mifepristone is the first of two drugs in a chemical abortion, the abortion method that has surpassed surgical abortion to be the most common in the U.S. through rampant ease of access and minimal oversight. The drug has been shown to be four times more dangerous than surgical abortion.
A groundbreaking analysis released in April of 865,000 insurance claims of women who were prescribed mifepristone abortions from 2017 to 2023 found that mifepristone is 22 times more dangerous than previously recognized in FDA data. The report by conservative think tank Ethics and Public Policy Center (EPPC) said that nearly 11% of women experience sepsis, infection, hemorrhaging, or another serious adverse event within 45 days of taking the drug.
The FDA uses is a drug safety program known as REMS (Risk Evaluation and Mitigation Strategies) for medications “with serious safety concerns” to make certain the benefits of the drug outweigh its risks. Of the 20,000+ prescription drugs approved by the FDA, just 74 drugs have been deemed dangerous enough to warrant a REMS restriction – and one of those is mifepristone.
Mifepristone was first approved in 2000 in a process that some contend was hurried, at odds with science and law, and pervaded by politics.
The FDA then loosened its own safety standards on the drug in 2016 under President Barack Obama and 2021 under President Joe Biden. Part of this reduction of safety standards has included eliminating the requirement that abortion providers report non-fatal adverse events to the FDA Adverse Event Reporting System.
The safeguards for mifepristone removed by the FDA over the last nine years include an initial in-person doctor visit to screen for ectopic pregnancy and other serious conditions, and a follow-up visit to check for life-threatening complications, such as internal bleeding and infection.
The FDA then further lessened its REMS requirements for chemical abortion drugs, permitting them to be dispensed by pharmacies and delivered via mail. The Biden administration made access to the drug without in-person dispensing permanent in 2023.
FDA data shows that at least 32 women have died following mifepristone use between the drug’s approval in 2000 and 2022.
The head of the largest network of pregnancy help organizations in the U.S. said that Kennedy’s admission before the Senate Finance Committee was not surprising and questioned whether the Trump administration would correct the FDA data for the good of women.
“It is no surprise to learn an abortion politician has intentionally twisted data,” said Jor-El Godsey, president of Heartbeat International. “They’ve been doing this for years with the other researched aspect of abortion by denying the negative effects on women.”
“The bigger question,” Godsey said, “is once finding it, will this administration have the courage to correct the analysis and act to protect the public?”
Heartbeat International’s Director of Government Relations noted the Biden administration’s handling of the FDA data on mifepristone put women at risk.
“We’re sad but not surprised to learn that the Biden FDA deliberately twisted the data and turned a blind eye to details about how abortion drugs have hurt women,” Jessica Prol Smith said.
Heartbeat International had submitted an amicus brief in FDA v. Alliance for Hippocratic Medicine, the Supreme Court case where a group of pro-life doctors and organizations had challenged the FDA’s decision to remove its health and safety measures for mifepristone, SCOTUS ultimately ruling that the doctors did not have standing – but upholding their conscience rights and refraining from ruling on the merits of the case.
Heartbeat had argued in its brief that the FDA put women at risk by lowering the standard of care for treating pregnant mothers when it relaxed its own rules for chemical abortion drugs.
Heartbeat International submitted an amicus brief in GenBioPro v. Raynes as well, in support of the state of West Virginia’s right to regulate chemical abortion pills.
Among the more minor side effects possible with mifepristone are cramping, nausea, and vomiting. Severe complications that require immediate medical attention include extremely heavy bleeding, severe infection, and retained tissue.
Aside from the risks, many chemical abortions result in the mother left to deliver her deceased child at home or in other circumstances without medical oversight, and quite often women have not been prepared for this.
Pro-life concerns also include the likelihood for the drug to be used in human trafficking and other situations involving abuse or coercion.
Kennedy said earlier this year on the day of his confirmation as HHS Secretary that President Donald Trump had asked him to study the safety of mifepristone. In May following release of the EPPC study Kennedy said the FDA should change the label on mifepristone and perform a top-to-bottom review of the drug.
FDA Commissioner Dr. Marty Makary had said during his confirmation hearing in March that he would take a “solid, hard look” at the data on mifepristone. After stating in April he had no plans to take action on mifepristone Makary confirmed in June the FDA would conduct a review of mifepristone.
During the Sept. 4 Senate Finance Committee hearing, Kennedy pledged to keep Lankford and Montana Sen. Steve Daines apprised of the FDA review.
Daines had called the claims that mifepristone is as safe as Tylenol “misleading” and “very harmful,” and questioned Kennedy about reinstating the in-person prescribing requirement.
Prol Smith praised the pro-life lawmakers and called on the FDA to protect women in keeping with the agency’s purpose.
“We’re grateful to see pro-life Senators Daines and Lankford providing oversight, and we join them in asking this FDA to do its job,” she said. “We know that health research and policy seem constantly controversial. But American women simply deserve better than the lies and half-truths they’ve gotten from abortion advocates who happen to hold government jobs.”
Lisa Bourne is Managing Editor of Pregnancy Help News and Content Writer for Heartbeat International.
Image: HHS Sec. Robert F. Kennedy/Susan B. Anthony Pro-Life America Facebook.
