
The Abortion Pill Harms Women: Insurance Data Reveals One in Ten Patients Experiences a Serious Adverse Event
By: Jamie Bryan Hall and Ryan T. Anderson, originally published April 28, 2025, Ethics & Public Policy Center
Summary
- This largest-known study of the abortion pill is based on analysis of data from an all-payer insurance claims database that includes 865,727 prescribed mifepristone abortions from 2017 to 2023.
- 10.93 percent of women experience sepsis, infection, hemorrhaging, or another serious adverse event within 45 days following a mifepristone abortion.
- The real-world rate of serious adverse events following mifepristone abortions is at least 22 times as high as the summary figure of “less than 0.5 percent” in clinical trials reported on the drug label.
- The FDA should immediately reinstate its earlier, stronger patient safety protocols to ensure physician responsibility for women who take mifepristone under their care, as well as mandate full reporting of its side effects.
- The FDA should further investigate the harm mifepristone causes to women and, based on objective safety criteria, reconsider its approval altogether.
Danco Laboratories markets Mifeprex as “the safe and effective abortion pill,” but our research shows that mifepristone abortion, as currently practiced in the U.S., is not safe and effective. The manufacturer and the FDA rely on the results of 10 clinical trials with a total of 30,966 participants, less than 0.5 percent of whom reportedly experienced serious adverse reactions. In contrast, we analyzed real-world insurance claims data for 865,727 prescribed mifepristone abortions, broadly representative of women who obtain mifepristone abortions in the U.S. today, and we find a serious adverse event rate of 10.93 percent—at least 22 times as high as the summary figure reported on the drug label. In light of this research, we urge the FDA to reinstate earlier, stronger patient safety protocols and reconsider its approval of mifepristone altogether. Women deserve better than the abortion pill.
Our Key Findings
10.93 percent of women experience sepsis, infection, hemorrhaging, or another serious or life-threatening adverse event within 45 days following a mifepristone abortion, far greater than the summary figure of “less than 0.5 percent” in clinical trials reported on the drug label. Figure 1.
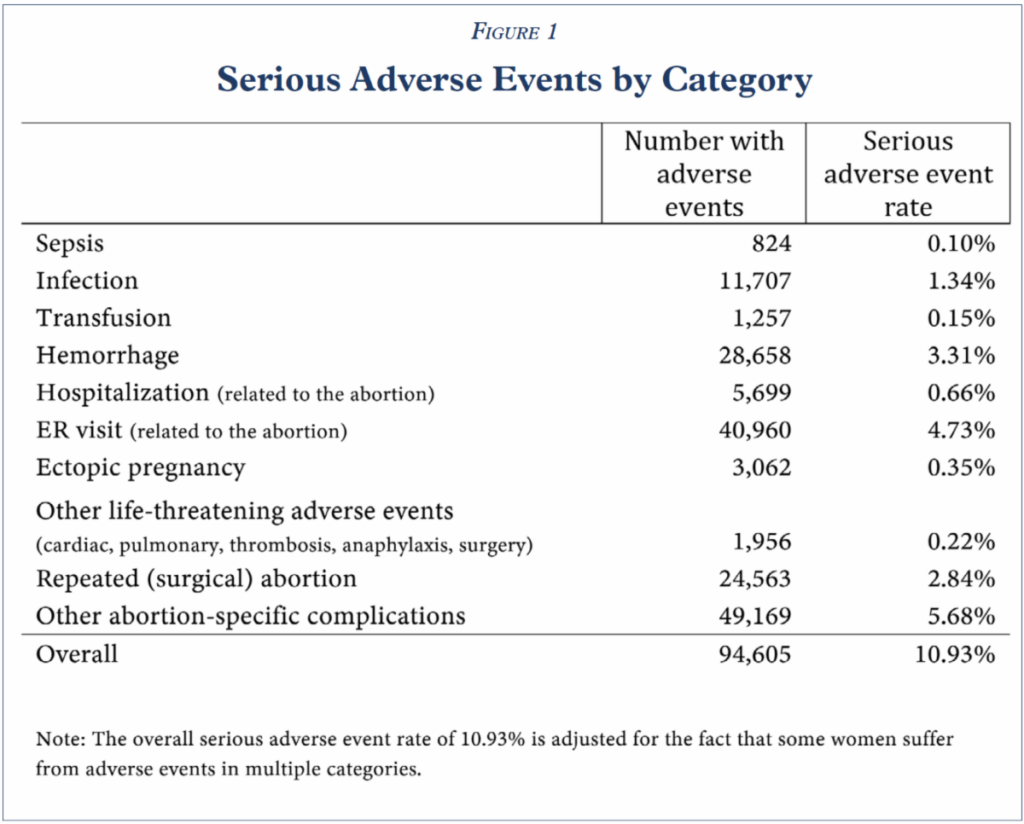
Simply stated, mifepristone, as used in real-world conditions, is not “safe and effective.”
- Our real-world post-market observational study of mifepristone is, to our knowledge, the most comprehensive study of chemical abortion safety ever conducted in the U.S.:
- We identify and include 865,727 prescribed mifepristone abortions, 28 times as many as were included in all FDA-cited clinical trials combined.
- Our dataset is more recent, no earlier than 2017, while the FDA approval of mifepristone relies entirely on data from more than a decade ago.
- The women in our dataset are broadly representative of the women who obtain mifepristone abortions in the U.S.; they are not a prescreened group of generally healthy women recruited into various clinical trials conducted at different times around the world.
- The women in our dataset receive (or fail to receive) pre- and post-abortion healthcare of the real-world quality that prevails in the U.S. today, not the carefully controlled regimen of care that ordinarily prevails in a clinical trial.
Policy Implications
The FDA should reinstate the original patient safety protocols that were required when mifepristone was first approved. Doing so will likely reduce the harms to women and permit better monitoring to determine whether this drug should remain on the market:
- Prescribing mifepristone and misoprostol for the termination of pregnancy should require at least three in-person office visits by the patient—as originally required by the FDA.
- Mifepristone should be prescribed only by physicians—as originally required by the FDA—who have read and understood the prescribing information.
- Mifepristone should be administered only in a clinic, medical office, or hospital, by or under the supervision of a physician, able to assess the gestational age of an embryo and to diagnose ectopic pregnancies—all of which was originally required by the FDA.
- Physicians must be able to provide surgical intervention in cases of incomplete abortion or severe bleeding, or have made plans to provide such care through others, and be able to assure patient access to medical facilities equipped to provide blood transfusions and resuscitation, if necessary.
- Healthcare providers should be required once again to report to the FDA (and manufacturers of mifepristone) all serious adverse events resulting from the use of mifepristone.
- Mifepristone should only be prescribed to a woman who is confirmed by a physician to be in the first seven weeks of pregnancy—as originally required by the FDA.
Background on FDA Approval and Regulation of Mifepristone
Mifepristone was developed by the French pharmaceutical company Roussel Uclaf S.A. and brought to the United States at the urging of President Clinton and after Congressional pressure. The FDA approved it under a little-used approval process for “certain new drug products that have been studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit to patients over existing treatments.” Thus, to grant approval in this manner, the FDA had to consider an unwanted pregnancy a “serious or life-threatening illness” and find that mifepristone was more effective than surgical abortion in treating it. The FDA’s decision cites minimal data: clinical trials with only 859 participants in the U.S. and 1,800 in France.
The original FDA-approved drug label for Mifeprex (the brand name of mifepristone) from September 2000 indicated use of the drug for “the medical termination of intrauterine pregnancy through 49 days’ pregnancy.” It required several modest safeguards for women’s health:
Treatment with Mifeprex and misoprostol for the termination of pregnancy requires three office visits by the patient. Mifeprex should be prescribed only by physicians who have read and understood the prescribing information. Mifeprex may be administered only in a clinic, medical office, or hospital, by or under the supervision of a physician, able to assess the gestational age of an embryo and to diagnose ectopic pregnancies. Physicians must also be able to provide surgical intervention in cases of incomplete abortion or severe bleeding, or have made plans to provide such care through others, and be able to assure patient access to medical facilities equipped to provide blood transfusions and resuscitation, if necessary.
During the Obama and Biden administrations, the FDA chipped away at these initial safeguards, risking women’s health in order to increase access to abortion. Under the current Risk Evaluation and Mitigation Strategy (REMS) in effect since 2023:
- a mifepristone abortion now requires as a little as one telehealth visit with any approved healthcare provider (not necessarily a physician),
- a woman may self-administer drugs obtained from a mail-order pharmacy, and
- the prescriber need not report any adverse events unless he or she knows that a patient has died. Figure 2.
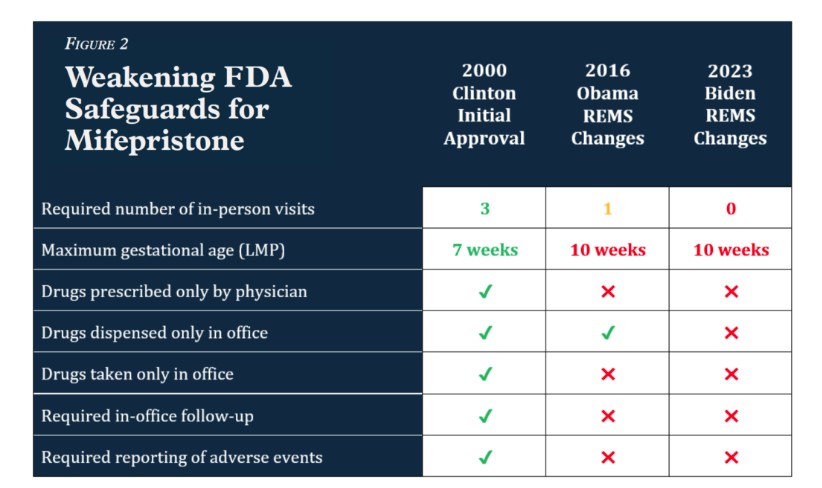
The current FDA-approved drug label refers to the results of 10 clinical trials with a total of 30,966 participants, less than 0.5 percent of whom reportedly experienced serious adverse reactions. But these figures are based entirely on data from trials taking place more than a decade ago.
The Increasing Market Share of Chemical Abortion
Increased access to chemical abortion has coincided with a rapid increase in its prevalence. Chemical abortions—the vast majority of which are performed using a combination of mifepristone and misoprostol—now account for roughly two-thirds of all abortions in the United States. Figure 3.
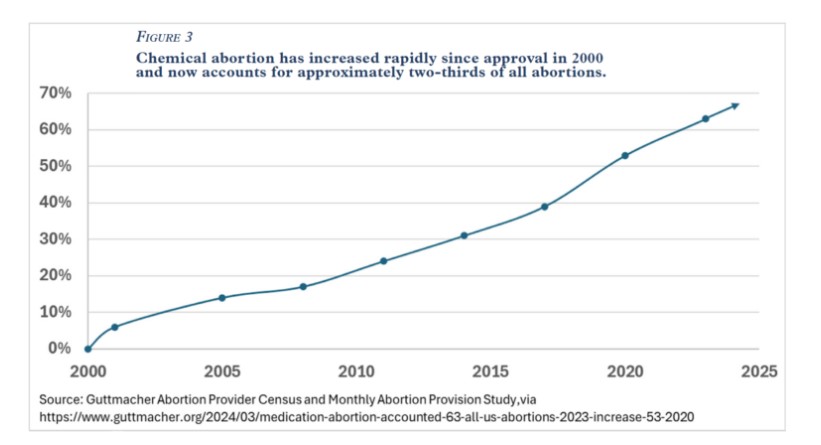
In fact, Danco Laboratories boasts that more than 5 million U.S. women have used its abortion pill since it was approved in 2000. Thus, it has become increasingly important to understand the risks and harms to women from chemical abortion in general and from mifepristone in particular.
How We Identified Mifepristone Abortions
In our data, a mifepristone abortion was identified in one of three ways:
- procedure code S0199: medically induced abortion by oral ingestion of medication including all associated services and supplies,
- a prescription for mifepristone (with or without misoprostol within the next 3 days), or
- a diagnosis code for elective termination or unwanted pregnancy, along with other billing coding consistent with manufacturer instructions for reimbursement for a mifepristone abortion for a given state and insurer combination.
Following this method, we identified a total of 865,727 mifepristone abortions prescribed for a total of 692,873 women from 2017 to 2023. This includes 566,446 women, each of whom had one such abortion, and 126,427 women with multiple such abortions.
How We Identified Serious Adverse Events
We followed the official FDA definition of “serious adverse event.” To do so, we identified relevant ICD-10 diagnosis codes and CPT/HCPCS procedure codes for adverse events from multiple sources:
- CDC Severe Maternal Morbidity (SMM) indicators,
- CMS Diagnosis-Related Group (DRG) 770 and 769 codes,
- FDA Adverse Events Reporting System (FAERS) reports (interpreted and translated into diagnosis and procedure codes by the doctors on our team), and
- a small number of (mostly gynecological) codes suggested by our doctors.
These codes were then graded by a team of physicians using the the NIH Common Terminology Criteria for Adverse Events (CTCAE version 5), which categorizes adverse events by severity on a 5-point scale. We included CTCAE Grade 3 (severe) and Grade 4 (life-threatening). We did not include Grade 1 (mild) or Grade 2 (moderate). (Grade 5 is death, which would count as a serious adverse event under the FDA definition but could not necessarily be observed in the insurance claims data. We hope to show the rate of death in a subsequent report.)
For reporting, we have grouped the relevant diagnosis and procedure codes as follows:
- The mifepristone label divides serious adverse events into six categories identified in the clinical trials: sepsis, infection, transfusion, hemorrhage, hospitalization, and emergency room visit. Only those hospitalizations and emergency room visits with abortion-related diagnosis and procedure codes are included in our analysis.
- FAERS received reports of various events that our doctors judge to be life-threatening and have grouped into broad categories: ectopic pregnancy, surgery, cardiac, pulmonary, thrombosis, and anaphylaxis.
- Repeated (surgical) abortion procedures are considered serious adverse events.
- Other abortion complications include codes specifically related to an abortion or miscarriage, as well as life-threatening mental health diagnoses, etc.
We consider only serious adverse events that occurred within 45 days following the abortion. This is conservative, as some adverse events may present later (and studies relied on by the FDA used a timeframe as long as 72 days). Additionally, the likelihood of a subsequent pregnancy within this timeframe is negligible, which ensures that adverse events are related to the abortion rather than to a subsequent pregnancy.
Our Data
Our team has purchased access to a commercially available all-payer health insurance claims database including de-identified data for all U.S. patients during the years 2017 to 2023. It includes information on hospital and office visits, diagnoses, procedures, and prescriptions processed through private health insurance, Medicaid, Medicare, TRICARE, and the Department of Veterans Affairs (VA). The data excludes transactions for which the insurer is also the provider (as is the case with some HMOs and much VA care), as well as cash pay transactions (which are disproportionately common for abortion). This dataset provides longitudinal tracking of medical diagnoses, procedures, and prescriptions for each patient and is widely used in academic and regulatory research.
Our Research Project Team
Our research project was conducted and validated by a team of data scientists, analysts, and engineers, with assistance from our clinical team of board-certified obstetricians and gynecologists. Members have a history of academic research and peer-reviewed publication.
Conclusion
Our research shows unequivocally that mifepristone abortion, as currently practiced in the U.S., is considerably more dangerous to women than is represented on the FDA-approved drug label. The FDA should immediately reinstate its earlier, stronger patient safety protocols to ensure physician responsibility for women who take mifepristone under their care, as well as mandate full reporting of its side effects. The FDA should further investigate the harm this drug causes to women and, based on objective safety criteria, reconsider its approval altogether. Women deserve better than the abortion pill.
This paper is the first in a series investigating women’s health and abortion using real-world data.
Ryan T. Anderson, Ph.D., is the President of the Ethics and Public Policy Center.
Jamie Bryan Hall is the Director of Data Analysis and a fellow in the Life and Family Initiative at the Ethics and Public Policy Center.
